Study Details Overview
The Study Details tab in Ripple is a centralized section designed for capturing and managing key information about your study. The data entered here plays a critical role in generating reports, such as enrollment metrics and study summaries, to monitor and evaluate study progress effectively.
- Navigate to the Study Settings tab (Red Section).
- Navigate to the Details section (Green Section).
By keeping the Study Details tab up-to-date, you can streamline the creation of reports and maintain a comprehensive record of your study’s key details.
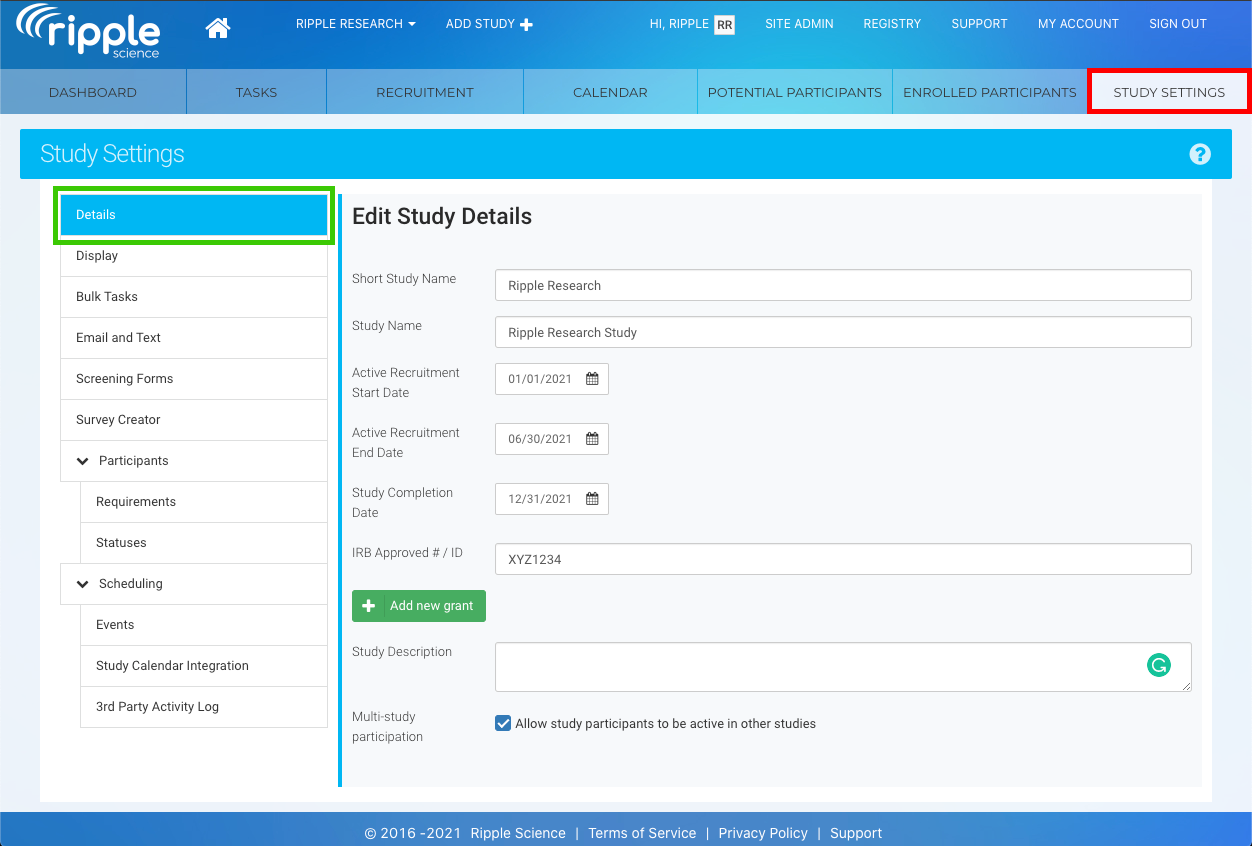
Study Details Fields in Ripple
The Study Details section is essential for managing key study information in Ripple. Below is an explanation of each field and its significance:
1. Study Short Name
-
- Purpose: A brief, 15-character name used to identify your study throughout Ripple.
- Usage:
- Appears in tags, such as participant comments marked as "Global."
- Ensures quick recognition of the study in Ripple's interface.
- Example: "HeartStudy" for a cardiovascular study.
2. Study Name
-
- Purpose: The full, official name of your study, without character limits.
- Behavior:
- Automatically populated with the Study Short Name if left blank.
- Used to provide the complete name if the short name is insufficient.
- Example: "Comprehensive Study on Cardiovascular Health Trends."
3. Active Recruitment Start/End Dates
-
- Purpose: Defines the timeframe for participant recruitment.
- Functionality:
- Recruitment progress metrics on the Dashboard are based on these dates.
- Helps track study enrollment timelines.
- Example:
- Start Date: January 1, 2025
- End Date: December 31, 2025
4. Study Completion Date
-
- Purpose: Marks the official end of the study when all participant events are complete.
- Significance: Indicates that the study is no longer active.
5. IRB Approved #/ID
-
- Purpose: Records the Institutional Review Board (IRB) number or ID, if applicable.
- Significance: Essential for studies requiring ethical review and approval.
6. Study Description
-
- Purpose: A text field for adding detailed study information.
- Examples of Content:
- Abstract or study summary.
- Inclusion and exclusion criteria.
Important Note: Information entered here is stored only in the "Study Details" section and is not visible elsewhere in Ripple.
7. Multi-Study Participation
Purpose: Manages participant eligibility for other studies in Ripple.
Checkbox Options:
✅ Checked: Active participants can be enrolled in multiple studies simultaneously.
❌ Unchecked: Active participants in this study cannot be added to other studies.
